MEGGLE - high quality crystalline lactose products, designed for DPIs.
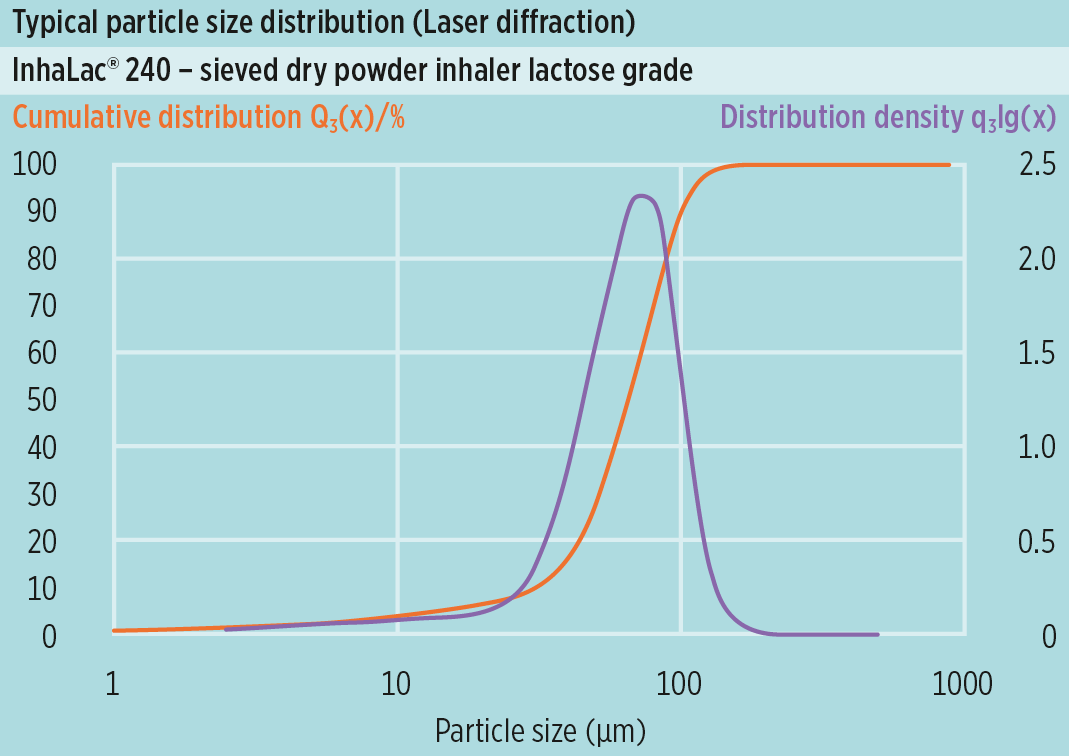
The new Inhalac® 240 is a sieved grade with a fine median particle size of approx. 65μm, but still fair flowability characteristics (Carr Index), which makes it suitable for reservoir devices.
InhaLac® 240 expands the product line of sieved lactose grades for dry powder inhalation.
Inhalation aerosols offers the potential for needle-free systemic delivery of small molecule drugs as well as therapeutic peptides and proteins. An industry standard in dry power inhalation formulation development, lactose monohydrate is used safely in DPI formulations. Using lactose as an excipient not only improves performance efficiency of the inhaler, but also facilitates powder handling during production. Effective performance of a dry powder inhaler largely depends on the carrier material used for the drug formulation.
With our highly controlled production process different high quality sieved and milled grades are obtained, providing an adequate range of particle sizes – making them behave as individual particles.
MEGGLE always at your service.
Phone: +49 (0) 8071-73-476
Shelf life / Retest:24 months
Standard Packaging:25 kg - Carton box with double PE-EVOH-PE Inliner